Monthly Archives: April 2018
Management of Uterine Fibroids

For over 100 years, surgery was the mainstay of treatment of uterine fibroids or myomas. Up until recently, therapeutic management of symptomatic uterine fibroids was broadened to three pillars: surgery, pharmacotherapy, and interventional radiotherapy. Nearly 50% of hysterectomies done for benign reasons are due to myomas.1
American data reveals that myomas occur in about 60% of African-American women and in upto 40% or Caucasian women aged 35. The incidence goes even higher at age 50 to around >80% in Black women and 70% of Caucasian women. Sex hormones have been implicated to cause the growth of myomas.2 Thus, when a woman is approaching menopause which is at an average of 49 or 50 among Filipinos, the drop in hormones may actually save her from hysterectomy depending on the size of the myoma and severity of symptoms such as bleeding and pain.
SIGNS & SYMPTOMS. Signs and symptoms of myomas vary. More than size, location of the myomas in the uterus vary since very large myomas up to 16-20 cm located at the outermost layer of the uterus called the serosa, have gone unnoticed, especially if the patient is obese. Conversely, even a 1 cm. myoma can cause vaginal bleeding if located in the cavity of the uterus. The patient may also experience pelvic pain if the myoma causes pressure on the nearby organs. If a bulky tumor grows forward and applies pressure on the urinary bladder in front it, the patient may experience frequency of urination, or inability to completely empty the bladder or inability to sleep due to constant bathroom trips. If the bulky tumor presses on the back of the uterus, it can impinge on the intestines and cause constipation. Myomas may also cause infertility and frequent miscarriage. Myomas or polyps are found in nearly 30% of women with recurrent miscarriage. 3,4
DIAGNOSIS. The gold standard for diagnosis is still the transvaginal ultrasound for non-virgins, or transrectal ultrasound in virgins. However, if the uterus rises above the pelvis and may be too large for the vaginal probe to reach, a pelvic or transabdominal ultrasound is done. A Magnetic Resonance may also help visualize the position, relations to the adjacent structures and actual number.
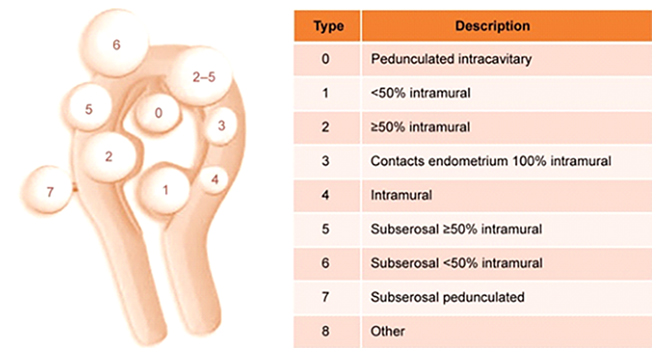
Figure 1
The International Federation of Gynecology and Obstetrics classification system for uterine fibroids.5
FIGO classification of uterine fibroids according to Munro et al. (2011). Fibroid types range from 0 to 8. 0 = Pedunculated, intracavitary; 1 = Submucosal, <50% intramural; 2 = Submucosal, ≥50% intramural; 3 = Contact with endometrium, 100% intramural; 4 = Intramural; 5 = Subserosal, ≥50% intramural; 6 = Subserosal, <50% intramural; 7 = Subserosal, pedunculated; 8 = Other (e.g. cervical, parasitic). Where two numbers are given (e.g. 2–5), the first number refers to the relationship with the endometrium, while the second number refers to the relationship with the serosa; e.g. 2–5 = Submucosal and subserosal, each with less than half the diameter in the endometrial and peritoneal cavities respectively. Fibroid classification cartoon republished with permission from Munro et al. (2011).
CONSERVATIVE MANAGEMENT
Patients who present with bleeding were usually offered tranexamic acid for 3-4 days monthly. It is a drug that promotes coagulation that has has been proven to reduce blood loss during menstruation. It is very well tolerated with few side effects.6,7.
The levonorgestrel intrauterine (LNG-IUS) has also been used successfully in bleeding patients with myomata.8 However, the presence of a submucous myoma distorting the uterine cavity may prevent a gynecologist from actually inserting the device properly.
Nonsteroidal anti-inflammatory drugs (NSAIDs) are also commonly used especially for patients with pain/and or bleeding in addition to hormones, either estroprogestins or progestogens, acting on the endometrium. Although it does not directly affect the fibroid to shrink it, it can reduce menstrual blood loss in women with mild and even severe bleeding. It is well tolerated as long as taken with food.9
Until 2012, GnRH agonists were the only therapeutic drugs approved for the treatment of myoma. The use of GnRH analogues reduces fibroid size and
bleeding symptoms. They act by reducing estrogen concentrations in the blood serum to postmenopausal levels. The side effects may be undesirable since it causes menopausal symptoms like hot flushes, skin and vaginal dryness, mood swings, loss of libido, vaginosis, depression, and bone loss. GnRH agonists are usually administered as subcutaneous injections for 3-4 months prior to surgery and they are not recommended for long-term use.
However, GNRH agonists have been losing ground because of their side effects such as hot flushes and bone loss plus the advent of a newer, cheaper drug that can be taken orally instead of subcutaneous injection. 10The new drug is ulipristal acetate (UPA), a selective progesterone receptor modulator oral drug, which can cause the bleeding from myomas to stop after 7 days of use and up to a 51% reduction in fibroid size after 13 weeks of use. By giving UPA tablets prior to Laparoscopic or Robotic surgery of large masses, shrinkage of the mass may actually happen with less possibility for anemia and blood transfusion since the bleeding pre-operatively may also be stopped. Another advantage of this drug versus the GNRH agonists is that the blood levels of estrogen remain higher similar to the estrogen levels women have in the first half of the menstrual cycle. This therefore means that there are less side effects such as thromboembolism and osteoporosis which are inherent to GNRH agonists. Side effects with multiple treatments of UPA are usually headaches, nasal congestion and sore throat, abdominal pain, and hot flushes. Because a greater number of
progesterone receptors have been found in myomata compared to normal uterine tissue another advantage of UPA is that it induces cell death or “apoptosis”. Therefore, unlike GNRH agonists, where the myoma shrinks only while you are taking the drug then grows back again to the previous size when you stop the drug, with UPA, the fibroid shrinkage persists for 6 mos. after stopping the drug. UPA, however, causes thickening of the lining of the uterus. Therefore, the drug is administered for three months then rest for two months before resuming the drug for three months again. 12
However, following recent reports of 4 women who developed severe liver failure while on UPA, 3 ending up with a liver transplant, on February 2018 the Medicines & Healthcare products Regulatory Agency (MHRA) advised of new temporary safety measures for ulipristal acetate13 following reports of serious liver injury in women using the medicine for uterine fibroid
The temporary safety measures are:
- Not to initiate new prescriptions for ulipristal acetate even for women who have already one or two courses of the drug previously.
- Monitor all women on the drug with liver function tests at least once a month. Stop UPA treatment in any woman who develops transaminase levels more than 2 times the upper limit of normal, closely monitor and refer for specialist hepatology evaluation as clinically indicated. Repeat liver function tests in all women 2 to 4 weeks after stopping treatment.
- For any recent or present users of UPA with signs or symptoms suggestive of liver injury (such as nausea, vomiting, malaise, right hypochondrial pain, anorexia, asthenia, jaundice), check transaminase levels immediately, If transaminase levels are more than 2 times the upper limit of normal, stop treatment, closely monitor and refer for specialist hepatology evaluation as clinically indicated.
- Advise women using UPA on the signs and symptoms of liver injury.
UTERINE ARTERY EMBOLIZATION
Uterine artery Embolization is a procedure where small polyvinyl particles are delivered via a catheter to the uterine arteries inserted on the femoral artery by an interventional radiologist. By doing so, the blood supply to the uterine fibroid is blocked, resulting in shrinkage, not disappearance of the myoma. It is indicated in women who have a major contraindication to undergo a surgery, or in women who strongly want to keep their uterus. Although it is considered a fertility-sparing procedure, it is, not recommended if a woman is desirous of pregnancy since she may actually drop her ovarian reserve.
Some of the side effects may include pain which results from occluding the blood supply, fever, infection and sepsis. The data also shows that if one resorts to a uterine fibroid embolization, the chances are higher that a patient may require a further surgery in two years which compared to women who underwent a straight myomectomy or an outright hysterectomy.14
HIGH INTENSITY FOCUSED ULTRASOUND (HIFU)
In contrast to Uterine Artery Embolization, HIFU uses no radiation. Rather, it acts much like the principle of using the magnifying glass to focus sunlight and burn paper. Focused ultrasound makes use of an acoustic lens to concentrate multiple intersecting beams of ultrasound on a target. Each single beam can pass through tissues with little effect but at the focal point where the beams all converge, the energy can generate heat high enough to ablate fibroids and cause cell death, blast ovarian cysts and even get rid of kidney stones.
On Oct. 2004, the US FDA first approved the HIFU for treatment of symptomatic uterine fibroids. The HIFU has been shown to be a safe and effective morality in achieving symptomatic relief while avoiding the risks inherent to surgery or other invasive therapies.15 Upto 16-20% will require additional intervention. 13
SURGICAL TREATMENT HYSTEROSCOPY
Hysteroscopic enucleation of a myoma is possible if the location of the myoma is inside the uterine cavity, the submucosal type. The patient usually comes to the clinic with complaints of bleeding. In fact, 59.8% of patients with myoma who present with vaginal bleeding actually have a submucosal myoma.16 It may also be discovered in patients who cannot get pregnant and in those with recurrent spontaneous abortion.17,18 Patients who have submucosal uterine fibroids may be attributed to dysregulated uterine contractility.19
In 20% of infertile patients, intrauterine fibroids or polyps are diagnosed.17,20 After hysteroscopic resection of intrauterine myoma, pregnancy rates
reach about 50%21 and bleeding symptoms are resolved in 70-99% of cases.18
As previously presented, we go back to the International Federation of Gynecology & Obstetrics classification of myoma:
• Type 0: the myoma is located completely in the uterine cavity or pedunculated.
• Type 1: <50% of the myoma is located in the myometrium.
• Type 2: >50% of the myoma is located in the myometrium.
It is important to determine this in order to predict the possibility for complete resection of the myoma prior to the procedure. According to European Society of Hysteroscopy (ESH) in 2012, this is the percentage success rate for complete resection of a myoma:
• Type 0: 96-97%
• Type 1: 86-90%
• Type 2: 61-83%
The success of surgery & rate of complete resection correlates inversely with the size of the myoma.22 An infertile patient diagnosed with a submucous myoma, even if it is less than 1.5 cm in size and without symptoms, must be subjected to a hysteroscopic myomectomy because the chances of success in pregnancy are high. In dealing with Type 2 myomas larger than 4 cm in size, a higher level surgeon expertise and experience is required since the complication rate increases with longer operating time. Since fluid is constantly infused into the uterine cavity in the course of the hysteroscopic myomectomy, there is a risk for fluid overload that can be fatal if unmonitored. 23
Hysterectomy

Hysterectomy is resorted to when fertility is no longer desired and/or if the patient is between 42-50 and wants a definitive treatment for her uterine tumor/s. Hysterectomy is also to be performed in a patient in the perimenopause or menopausal age where cancer is either suspected or verified. The surgery may be done by traditional open surgery, laparoscopy or robotic surgery. However, in the last decade, minimally invasive surgery by laparoscopy or robotic surgery has largely replaced open surgery in centers where the machine is available and where credentialed gynecologists are present to perform the surgery.
Laparoscopy reaches its technical limits depending on the experience and skill of the laparoscopist as well as the technical difficulty of a case, like massive adhesions plus economic considerations for the patient. Surgeons with much experience in removing very large uteri will not hesitate to remove uteri with myomas of over 7 cm; whereas, the average laparoscopic surgeon may use 5-7 cm as a limit, beyond which, they will prefer to first shrink the mass with GNRH antagonists or ulipristal acetate, or just perform an open surgery. Laparoscopy requires instilling carbon dioxide gas into the abdominal cavity to better see the entire area up to the liver and diaphragm, while under general anesthesia. Because of this, certain subsets of patients with morbid obesity or cardiovascular symptoms cannot tolerate this set up and may be better with an open procedure.32
There is data that shows that post-operative pain intensity among post robotic hysterectomy patients is less compared to post laparoscopic hysterectomy patients. This may be because with lap surgery, the pivot point of the lap instruments is the abdominal wall itself, whereas robotic instruments pivot at a remote, fixed center of motion away from the abdominal wall, thereby decreasing the injury to the anterior abdominal wall itself.33 Some authors, though, found no difference in pain or narcotic use post-op between patients who underwent a laparoscopic hysterectomy vs robotic hysterectomy. 34
In conclusion, myomas are benign tumors that afflict over half of women in their reproductive age presenting with various symptoms of pain, bleeding, pressure on the bladder and rectum. Treatment may be conservative or definitive, medical or surgical depending on the size, location of tumor, age of patient and desire for fertility.
- Wallach EE, Vlahos NF. Uterine myomas: an overview of development, clinical features, and management. Obstet Gynecol. 2004;104(2):393-406.
- Jacoby VL et al. Racial and ethnic disparities in benign gynecologic conditions and associated surgeries. Am J Obstet Gynecol. 2010;202(6):514-21.
- Islam MS et al. Uterine leiomyoma:available medical treatments and new possible therapeutic options. J Clin Endocrinol Metab. 2013;98(3):921-34.
- Bohlmann MK et al. Hysteroscopic findings in women with two and with more than two first-trimester miscarriages are not significantly different. Reprod Biomed Online. 2010;21(2):230-6.
- Int J Womens Health. 2017; 9: 607–617. Published online 2017 Sep 5. doi: 10.2147/IJWH.S138982Copyright/License
- Peitsidis P, Koukoulomati A. Tranexamic acid for the management of uterine fibroid tumors: a systematic review of the current evidence. World J Clin Cases. 2014;2(12):893–898. [PMC free article] [PubMed]
- Shabaan MM, Ahmed MR, Farhan RE, Dardeer HH. Efficacy of tranexamic acid on myomectomy-associated blood loss in patients with multiple myomas. A randomized controlled clinical trial. Reprod Sci. 2016;23(7):908–912. [PubMed]
- Senol T, Kahramanoglu I, Dogan Y, Baktiroglu M, Karateke A, Suer N. Levonorgestrel-releasing intrauterine device use as an alternative to surgical therapy for uterine leiomyoma. Clin Exp Obstet Gynecol. 2015;42(2):224–227. [PubMed]
- Jiang W, Shen Q, Chen M, et al. Levonorgestrel-releasing intrauterine system use in premenopausal women with symptomatic uterine leiomyoma: a systematic review. Steroids. 2014;86:69–78. [PubMed]
- Tosun AK, Tosun I, Suer N. Comparison of levonorgestrel-releasing intrauterine device with oral progestins in heavy menstrual bleeding (HMB) cases with uterine leiomyoma (LNG-IUD and oral progestin usage in myoma uteri) Pak J Med Sci. 2014;30(4):834–839.[PMC free article] [PubMed]
- Hoellen F et al. Therapeutic drugs in the treatment of symptomatic uterine broids. Expert Opin Pharmacother. 2013;14(15):2079-85.
- Schmidt T et al. Modi cations of laparoscopic supracervical hysterectomy technique signi cantly reduce postoperative spotting. J Minim Invasive Gynecol. 2011;18(1):81-4.
- Donnez J et al. Long-term treatment of uterine fibroids with ulipristal acetate. Fertil Steril. 2014;101(6):1565-73.e1-18
- https://www.rcog.org.uk/en/guidelines-research-services/guidelines/esmya-ulipristal-acetate—mhra-safety-alert/
- Gupta JK et al. Uterine artery embolization for symptomatic uterine broids. Cochrane Database Syst Rev. 2012;5:CD005073.
- Fennessy, Fiona; Fischer, Krisztina; McDannold, Nathan; Jolesz, Ferenc; Tempany, Clare (2015). “Potential of minimally invasive procedures in the treatment of uterine fibroids: a focus on magnetic resonance-guided focused ultrasound therapy”. International Journal of Women’s Health. 7: 901–12. doi:10.2147/IJWH.S55564. PMC 4654554 . PMID 26622192.
- Stewart, Elizabeth A.; Gostout, Bobbie; Rabinovici, Jaron; Kim, Hyun S.; Regan, Lesley; Tempany, Clare M. C. (2007). “Sustained Relief of Leiomyoma Symptoms by Using Focused Ultrasound Surgery”. Obstetrics & Gynecology. 110 (2, Part 1): 279–87. doi:10.1097/01.AOG.0000275283.39475.f6. PMID 17666601.
- Zimmermann A et al. Prevalence, symptoms and management of uterine fibroids: an international internet-based survey of 21,746 women. BMC Womens Health. 2012;12:6
- Bohlmann MK et al. Hysteroscopic findings in women with two and with more than two first-trimester miscarriages are not significantly different. Reprod Biomed Online. 2010;21(2):230-6
- 18. Capmas P et al. Surgical techniques and outcome in the management of submucous fibroids. Curr Opin Obstet Gynecol. 2013;25(4):332-8
- Aguilar HN, Mitchell BF. Physiological pathways and molecular mechanisms regulating uterine contractility. Hum Reprod Update. 2010;16(6):725-44.
- Hucke J et al. Hysteroscopy in infertility–diagnosis and treatment including falloposcopy. Contrib Gynecol Obstet. 2000;20:13-20.
- 21. Donnez J et al. Unusual growth of a myoma during pregnancy. Fertil Steril. 2002;78(3):632-3
- Bettocchi S et al. The destiny of myomas: should we treat small submucous myomas in women of reproductive age? Fertil Steril. 2008;90(4):905-10.
- Cravello L et al. [Results of hysteroscopic myomectomy]. Gynecol Obstet Fertil. 2004;32(9):825-8
- Kolankaya A, Arici A. Myomas and assisted reproductive technologies: when and how to act? Obstet Gynecol Clin North Am. 2006;33(1):145-52.
- Sunkara SK et al. The effect of intramural fibroids without uterine cavity involvement on the outcome of IVF treatment: a systematic review and metaanalysis. Hum Reprod. 2010;25(2):418-29
- Yan L et al. Effect of fibroids not distorting the endometrial cavity on the outcome of in vitro fertilization treatment: a retrospective cohort study. Fertil Steril. 2014;101(3):716-21.
- Yoshino O et al. Myomectomy decreases abnormal uterine peristalsis and increases pregnancy rate. J Minim Invasive Gynecol. 2012;19(1):63-7.
- Parker WH et al. Risk factors for uterine rupture after laparoscopic myomectomy. J Minim Invasive Gynecol. 2010;17(5): 551-4.
- Rooma S, Madhumati Sanjay, B. Rupa, and Samita Kumari. Robotic surgery in gynecology. J Minim Access Surg. 2015 Jan-Mar; 11(1): 50–59. doi: 10.4103/0972-9941.147690 PMCID: PMC4290120 PMID: 25598600
- Nezhat C, Lavie O, Hsu S, Watson J, Barnett O, Lemyre M. Robotic-assisted laparoscopic myomectomy: A retrospective matched control study. Fertil Steril. 2009;91:556–9. [PubMed]
- Bedient CE, Magrina JF, Noble BN, Kho RM. Comparison of robotic and laparoscopic myomectomy. Am J Obstet Gynecol. 2009 Dec:201(6):566.e1 5. Doi:10.1016/j.ajoj.2009.05.049.Epub2009 Aug 15.
- Hoellen F et al. Hybrid approach of retractor-based and conventional laparoscopy enabling minimally invasive hysterectomy in a morbidly obese patient: case report and review of the literature. Minim Invasive Ther Allied Technol. 2014;23(3):184-7
- Chiu LH, Chen CH, Tu PC, et al. Comparison of robotic surgery and laparoscopy to perform total hysterectomy with pelvic adhesions or large uterus. J Minim Access Surg 2015;11:87-93 .
- Turner TB, et al. Postoperative Pain Scores and Narcotic Use in Robotic-assisted versus Laparoscopic HysterectomyJ Minim Invasive Gynecol. 2015 Sept-Oct
Reverse Aging

It has been observed that the maximum life expectancy has risen by a quarter of a year, each
year for the past 160 years. Dr Jim Oeppen of Cambridge University, UK and Dr. James Vaupel
of the Max Planck Institute for Demographic Research in Germany predict that if the top life
expectancy will continue to increase by 2.5 years each decade, the world’s average life
expectancy should reach 100 within the next 50 years. Assuming the average age of
menopause is 49-50, this would mean, the women would live an equal amount of time of their
lives deprived of hormones, and if not managed, with rapid deterioration of their sexual function,
libido, osteoporosis (bones become brittle as a result of hormonal changes or vitamin or
mineral deficiency, osteoarthritis (degeneration of underlying bone and cartilage), sarcopenia
(loss of muscle tissue as a result of aging), and skin wrinkling.
There are women in their 20’s and 30’s according to their birth age who, because of poor diet
and lifestyle already have a metabolic age of a 50 or 60 year old person. They already are
candidates for hypertension, diabetes and cancer at such a young age. There are also women
who, once they reach menopause, already feel the degenerative effects of age and find their
weight suddenly uncontrollable, or who experience hot flushes, loss of libido, vaginal dryness,
skin dryness, thinning hair, mood swings, emotional lability manifested my crying spells,
insomnia, loss of zest for life. Reverse aging is about about optimizing the best version of
yourself and improving the quality of your life for your age by helping you look, feel and function
as well as possible. In contrast to conventional Medicine, which waits for illness to happen
before treatment is instituted, Reverse Aging is anticipatory and dynamic. Through a series of
tests and proactive treatment plans, adjustments are made with your diet, exercise,
supplements, with or without hormones, intended to preserve or restore your youth, as well as
your ability to enjoy your sexuality again.
Menopause Management


Menopause is the period in a woman’s life when her menses cease because the ovaries stop producing estrogen. Menopause marks the end of the reproductive years. It can happen naturally at an average of 51 years old. Menopause can also be induced as in surgical menopause when the ovaries are removed by bilateral oophorectomy, either alone or with the removal of the uterus. Menopause can also be induced when the ovaries are severely damaged by exposure to radiation, chemotherapy or other medications or toxins. Perimenopause are the years leading up to menopause. It may occur up to 10 years prior to menopause when the amount of estrogen produced by the ovaries begins to fluctuate.
How is Menopause Diagnosed?
According to the NICE Guidelines, if a woman is aged over 45 years and has not had a period for at least 12 months, or has vasomotor symptoms and irregular periods or who experiences symptoms after her uterus has been removed, this is adequate information to diagnose menopause even without the use of any laboratory tests. Hormonal tests should not routinely be used when diagnosing the menopause. In particular, the FSH test is deemed inappropriate for women taking combined hormonal contraception or a high-dose progestogen, nor should it be used for women aged over 45 years. Blood levels of FSH fluctuate markedly during the years leading up to menopause and they therefore do not help when forming what is actually a clinical diagnosis. The NICE Guidelines also does not advocate measuring the ff: antiMüllerian hormone (an indicator of ovarian reserve), inhibin A or B (which inhibit FSH production), estradiol, antral follicle count and ovarian volume. However, an FSH test should be considered for women aged 40–45 years with menopausal symptoms, including a change in their menstrual cycle, and in women under 40 years when the menopause is suspected, in whom premature ovarian insufficiency is a possibility. 1
What are the Signs and Symptoms of Menopause?
Common signs and symptoms include the following:
Vasomotor symptoms (VMS) such as hot flushes, cold sweats, night sweats, palpitations are experienced by peri- and menopausal women. A hot flush is a sudden feeling of heat that rushes to the upper body and face which may for a few seconds to several minutes. Some women have hot flushes a few times a month. Others have them several times a day. Hot flushes that happen at night (night sweats) may interrupt sleep and cause you to feel tired the following day. A cold sweat refers to sudden perspiration that does not come from heat or exertion, accompanied by a chill or cold sensation. All these symptoms may or may not be accompanied by palpitations which is the sensation that your heart is beating too hard or too fast.
- Sleep problems—Insomnia can manifest either as trouble falling asleep, or you may wake up in the middle of the night and being unable to sleep again. Night sweats can wake you and disturb your sleep.
- Vaginal and urinary tract changes—As estrogen levels decrease, the lining of the vagina may become thinner, dryer, and less elastic. Vaginal dryness may cause dyspareunia or pain during sexual intercourse. This may also increase the woman’s susceptibility to vaginal infections. The urethra can likewise become dry, inflamed, or irritate resulting in frequent urination and increased risk of urinary tract infections. 3
- Dryness of the skin – women experience dry skin in menopause as a result of the decline of estradiol, responsible partly for the growth and maintenance of blood capillaries in the dermis. With the decline in blood flow through the dermal capillaries, and less nutrients and oxygen are available to the the epidermis. This thins out the epidermis and slows the turnover rate of the cells, leading to increased trans-epidermal
water loss resulting in skin dryness - Acne & Facial hair– estradiol normally discourages acne formation by stimulating a more fluid sebaceous gland secretion during the reproductive years. However, because of the decline of estrogen in menopause, the testosterone produced by the adrenal glands is no longer masked. This results in a greater expression of testosterone in the woman’s body by stimulating sebaceous glands to secrete thicker sebum, giving the appearance
of oily skin which can manifest as acne in the perimenopause and menopausal period as well as abnormal facial hair, esp. in the chin area. - Wrinkling of the skin – In menopause, skin thickness decreases by 1.13% per postmenopausal year, with an associated decrease in collagen content (2% per post-menopausal year). 4 As the supportive fat below the skin of the face, neck, hand and arms diminishes and gets redistributed over the abdomen and/or on the thighs and buttocks, this results in sagging skin & wrinkles.
- Cerebral symptoms – some menopausal women may start complaining of headaches, migraines, anxiety, memory loss and difficulty in concentration which can all result from a drop in estradiol levels.
- Osteoarthritis – Osteoarthritis is the most common form of arthritis in adults and is characterized by radiographic and functional deterioration of joints. The most recognized feature is loss of articular bones, tendons, ligaments and muscles may also be commonly involved. Osteoarthritis is defined radiographically by findings of loss of joint space, the presence of bony spurs known as osteophytes, sclerosis of the subchondral
bone and bony cyst formation. Clinically, these findings on x-ray are associated with joint pain and stiffness in many patients typically involving large weight-bearing joints (such as the knee and hip), small joints (including the distal and proximal interphalangeal joints, the base of the thumb, and the big toe), and the cervical and lumbar spines. Patients typically complain of stiffness and difficulty in rising up from bed upon waking up
or arising from a chair after a long period of sitting or prolonged immobility, a symptom termed as gelling. Soft tissue swelling is not common, and more often bony deformities are found but large joint effusions can occur. 5 - Osteoporosis – the word actually means “porous bones.” As a result of this weakened structure from a loss of density, the risk of fractures increase, especially in the hip, spinal vertebrae, and wrist. Normally, bone tissue is constantly being renewed, and new bone replaces old, damaged bone. In this way, the body maintains bone density and the integrity of its crystals and structure.Bone density depends on how much bone was deposited via diet and activity till the person is around 25. Then from around 35 years and older, the bone density begins to decline since the bone eating cells are more active than the bone-depositing cell. Especially after menopause, without estrogen, the bone breaks down faster than it builds. This results in osteoporosis.
Acc to Ramoso-Jalbuena way back in 1994, In a survey of Filipino women aged 40-50 years, of various professions and residing mostly in Metropolitan Manila, the average age of menopause was estimated at 48 years. The climacteric symptoms were seen to affect 83% of the respondents. Sixty-three percent reported menopause-related circulatory or vasomotor disorders and 79% mentioned psychological disorders. The incidence and frequency of climacteric symptoms were highest among the perimenopausals. Headache was the most common climacteric symptom, while the hot flush was the least prevalent. Only 31% consulted a physician for menopause-related ailments. Eighty-six percent of those who consulted were prescribed medication, however, only 52% of these followed the prescription. Eleven percent
reported dyspareunia and only 36% consulted a doctor. Thirty-one percent suffered from urinary stress incontinence and only 16% consulted a doctor. The findings of this study suggest that the average Filipino woman has an attitude of forbearance towards the climacteric syndrome. 6 In a study was conducted in seven south-east Asian countries, namely, Hong Kong, Indonesia, Korea, Malaysia, the Philippines, Singapore and Taiwan, the median age at menopause (51.09) appeared to be within the ranges observed in western countries. 7 There is a school of thought that believes that menopausal symptoms are a peculiarly ‘Western’ phenomenon, not experienced by women from other regions and particularly not from Asia where, it has been claimed, dietary, social and cultural factors afforded protection for women living in that region. More recently, studies conducted in multi-ethnic communities living in Western countries as well as in Asian communities have found that the menopause and its consequences are similar world-wide. 8
Management:
For women experiencing vasomotor symptoms, do not routinely offer s elective serotonin
reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs) or
clonidine as first-line treatment. Instead, estrogen and progestogen therapy may be offered to
women with a uterus and estrogen therapy alone to women without a uterus. The short term (up
to 5 years) and longer-term benefits & risks must be discussed. For women afraid to take
hormones, explain to them that there is some evidence that isoflavones or black cohosh may
relieve vasomotor symptoms. However, multiple preparations are available, their safety is
uncertain & interactions with other medicines have been reported. For women with urogenital
atrophy, vaginal estrogen may be offered even if they are already on hormone replacement
therapy (HRT). 9
For vulvovaginal issues of menopausal women who are afraid to use hormones, ospemifene, a
tissue-selective estrogen receptor modulator (SERM), was recently approved for the treatment
of vulvovaginal atrophy and dyspareunia . Ospemifene relieves moderate to severe symptoms
of vulvovaginal atrophy, like dryness, irritation and soreness around the genital area, and painful
sexual intercourse, in menopausal women. It is well tolerated, and it has neutral effects on
endometrium and coagulation. Clinical trials and even long-term studies on breast cancer
effects support the overall safety of ospemifene. 10
In addition, women with urogenital atrophy may benefit from cutting-edge technology called
Laser Vaginal Rejuvenation. This involves the use of lasers to thicken the vaginal walls,
promoting the deposition of collagen and reversing the atrophy. This also results in more
transudation of fluid which promotes lubrication as well. Laser therapy may be valuable as a
nonhormonal therapeutic modality in the management of GSM. 11
The clinical effects of using hormone replacement therapy for a year or longer must be
discussed. This review included 22 double-blinded randomised controlled trials (RCTs) (43,637
women). The evidence is current to September 2016. 9
HRT is given for control of menopausal symptoms. It has also been used for the management
and prevention of chronic diseases such as cardiovascular disease, osteoporosis and dementia.
In relatively healthy postmenopausal women, using combined continuous HT for 1 year
increased the risk of a heart attack from about 2 per 1000 to between 3 and 7 per 1000, and
increased the risk of venous thrombosis (blood clot) from about 2 per 1000 to between 4 and 11
per 1000. With longer use, HRT also increased the risk of stroke, breast cancer, gallbladder
disease and death from lung cancer.
Estrogen-only HRT increased the risk of venous thrombosis after 1 to 2 years’ use: from 2 per
1000 to 2 to 10 per 1000. With longer use, it also increased the risk of stroke and gallbladder
disease, but it reduced the risk of breast cancer (after 7 years’ use) from 25 per 1000 to
between 15 and 25 per 1000.
Among women over 65 years of age taking continuous combined HRT, the incidence of
dementia was increased.
Risk of fracture was the only outcome for which results showed strong evidence of clinical
benefit from HRT (both types).
Women with intolerable menopausal symptoms may wish to weigh the benefits of symptom
relief against the small absolute risk of harm arising from short-term use of low-dose HT,
provided they do not have specific contraindications. HT may be unsuitable for some women,
including those at increased risk of cardiovascular disease, increased risk of thromboembolic
disease (such as those with obesity or a history of venous thrombosis) or increased risk of some
types of cancer (such as breast cancer, in women with a uterus). The risk of endometrial cancer
for women with a uterus who take oestrogen-only HT is well documented.
HRT is not indicated to prevent or treat cardiovascular disease or dementia, nor can it be used
for preventing deterioration of cognitive function in postmenopausal women. Although HT is
considered effective for prevention of postmenopausal osteoporosis, it is generally
recommended as an option only for women at significant risk, for whom non-oestrogen
therapies are unsuitable. Data are insufficient for assessment of the risk of long-term HT use in
perimenopausal women or postmenopausal women younger than 50 years of age.
Hormone therapy may be used to manage troublesome menopausal symptoms, but is currently
recommended to be given at the lowest effective dose and regularly reviewed by a woman and
her doctor. In women with an intact uterus, hormone therapy using estrogen and progestogen is
recommended to minimize the risk of endometrial hyperplasia , which can develop into
endometrial cancer. Low-dose estrogen plus progestogen (minimum of 1 mg norethisterone
acetate or 1.5 mg medroxyprogesterone acetate) taken daily (continuously) appears to be safe
for the endometrium. For women who had their last menstrual period less than one year ago
low-dose estrogen combined sequentially with 10 days of progestogen (1 mg norethisterone
acetate) per month appears to be safe for the endometrium . 12
In contrast to progestins, recent findings relating to the use of natural progesterone, are
reassuring. These findings come from two cohort studies carried out in France, where oral
micronized progesterone has been used by large numbers of menopausal women since over
two decades. 13
Th ere is good evidence that adding testosterone to hormone therapy (HT) has a beneficial
effect on sexual function in postmenopausal women. However, the combined therapy is
associated with a higher incidence of hair growth and acne and a reduction in high-density
lipoprotein (HDL) cholesterol. These adverse events may vary with different doses and routes of
administration of testosterone. Adding testosterone to HT did not increase the number of
women who stopped HT therapy . 14
For women with osteoporosis, management focuses first on nonpharmacologic measures, such
as a balanced diet, adequate calcium and vitamin D intake, adequate exercise, (a minimum of
40 mins/day brisk walking 2-3 times your normal walking pace), smoking cessation, avoidance
of excessive alcohol intake, and fall prevention. If pharmacologic therapy is indicated,
government-approved options are bisphosphonates, a selective estrogen-receptor modulator,
parathyroid hormone, estrogens, and calcitonin. 15
REFERENCES:
- https://www.nice.org.uk/guidance/ng23/chapter/Recommendations#diagnosis-of-perimen
opause-and-menopause - https://www.acog.org/Patients/FAQs/The-Menopause-Years .
- Brincat M, Moniz CJ, Studd JW, et al. Long-term effects of the menopause and sex
hormones on skin thickness. Br J Obstet Gynaecol. 1985;92:256–9. [ PubMed ]. - M. Julie Thornton , Dermatoendocrinol . 2013 Apr 1; 5(2): 264–270. Published online
2013 Apr 1. doi: 10.4161/derm.23872 - https://www.menopausemgmt.com/osteoarthritis-in-menopause/
- Ramoso-Jalbuena J ., Climacteric Filipino women: a preliminary survey in the Philippines .
Maturitas.1994 Oct;19(3):183-90. - Boulet MJ , Oddens BJ, Lehert P , Vemer HM , Visser A . Climacteric and menopause in
seven South-east Asian countries. Maturitas. 1994 Oct;19(3):157-76. - Baber, RJ. East is east and West is west: perspectives on the menopause in Asia and
The West. Climacteric . 2014 Feb;17(1):23-8. doi: 10.3109/13697137.2013.830607. Epub
2013 Oct 1. - http://www.cochrane.org/CD004143/MENSTR_long-term-hormone-therapy-perimenopau
sal-and-postmenopausal-women - Del Pup, L. Ospemifene: a safe treatment of vaginal atrophy. Eur Rev Med Pharmacol
Sci. 2016 Sep;20(18):3934-3944. - Arunkalaivanan A , Kaur H, Onuma O . Laser therapy as a treatment modality for
genitourinary syndrome of menopause: a critical appraisal of evidence. Int Urogynecol J.
2017 May;28(5):681-685. doi: 10.1007/s00192-017-3282-y. Epub 2017 Feb 2 - http://www.cochrane.org/CD000402/MENSTR_hormone-therapy-for-postmenopausal-wo
men-with-intact-uterus - Campagnoli , C., et al. Progestins and progesterone in hormone replacement therapy
and the risk of breast cancer. J Steroid Biochem Mol Biol. 2005 Jul; 96(2): 95–108. doi:
10.1016/j.jsbmb.2005.02.014 - http://www.cochrane.org/CD004509/MENSTR_testosterone-for-perimenopausal-and-pos
tmenopausal-women - North American Menopause Society .Management of osteoporosis in postmenopausal
women: 2006 position statement of The North American Menopause Society.
Menopause. 2006 May-Jun;13(3):340-67; quiz 368-9
Nutritional Therapy


Nutritional Therapy is an evidence-based approach to maximizing one’s health potential though individually formulated nutritional and lifestyle changes. It is premised on the basis that we have individual needs, lifestyles, eating habits and therefore, deficiencies, which may become the root of certain imbalances leading to disease. Therefore, by espousing the use of wholesome, unprocessed, non-inflammatory foods to promote optimal well-being, coupled with supplementation with nutraceuticals to compensate for the deficiencies, the root of whatever health oppressions one if afflicted with, can be addressed and even reversed.
There is no longer a “one size fits all” formula feeding for all people because of our genetic differences that alter the way we metabolize food, the production of certain enzymes, presence of absence of the insulin resistance gene, exposure to toxic substances, activities, geographic habitat and level of daily stress.
Nutritional Therapy treats the body on a wholistic basis while getting to the root cause of the disease or health concern, rather than simply treating or addressing the symptoms.
It can reverse, overweight and obesity, diabetes type II, metabolic derangements such as hypertension, elevated cholesterol, triglyceride uric acid, and fatty liver. Cancer can be prevented and post-cancer patients can prevent the recurrence of malignancies. Alopecia and androgenetic hair loss can be addressed and even reversed. Recurrent infections and auto-immune diseases can likewise be managed to diminish the frequency of flare. Infertile couples suddenly find themselves pregnant even after 8-10 years of infertility.

Weight Loss Management


Obesity is best defined by using the body mass index calculated using a person’s height and weight: the body mass index (BMI) equals a person’s weight in kg (KG) divided by the height in meters (m) squared. A normal BMI is between 18.5-24.9, An adult with a BMI of 25-29.9 is considered overweight and a person with a BMI of over 30 is considered obese.
Worldwide, the incidence of obesity has nearly doubled from 1991 to 1998.
Many patients are overweight even if they think they eat healthy. Sometimes, with some trigger like a changing to a night shift job or facing a stressful situation, a woman can suddenly find herself gaining unwanted kilos. Obesity does not only affect your appearance, it also affects your heart, your joints, your spine. Obesity is also linked to a higher risk for breast, colon, rectum, gallbladder, kidney, pancreatic and thyroid cancers. It is also the number one risk factor for developing sleep apnea. Because of the extra fatty tissue around the upper airway, this can make the airway smaller and likely obstruct. Obesity increases the risk for heart disease and hypertension. If you are preparing for pregnancy and are obese, it is best to drop your weight first. Pregnancy and obesity are both insulin resistant states. So, getting pregnant while obese
increases one’s propensity for gestational diabetes and hypertension in pregnancy, both very grave conditions that can lead to a growth retarded fetus and many other complications.
What are the causes of Obesity?
- Leptin Deficiency – Leptin is a hormone produced in fat cells as well as the placenta. This
hormone controls weight by signaling the brain to eat less when body fat stores are too high.
If, for some reason, the body cannot produce enough leptin or leptin cannot signal the brain
to eat less, this control is lost, and obesity occurs. This can be from a genetic cause. In fact,
if one parent is obese, more likely than not, the child can become obese. - A High Carb Diet – Carbohydrates convert to glucose (which is sugar) in the blood, which in
turn triggers the pancreas to secrete insulin. Insulin, in turn promotes the growth of fat tissue
that can cause weight gain plus, insulin also triggers the kidneys to retain sodium which
causes water retention. Simple carbohydrates like white rice, chocolates, sugars, fructose,
desserts, soft drinks, etc. contribute to weight gain because they are more rapidly absorbed
into the bloodstream than complex carbohydrates (pasta, brown rice, root crops, grains, raw
fruits, etc.) and thus cause a more pronounced insulin release after meals than complex
carbohydrates. - Physical inactivity. Sedentary people burn fewer calories than people who are active.
Physical inactivity can have serious implications for people’s health, Approximately 2 million
deaths per year are attributed to physical inactivity, prompting WHO to issue a warning way
back in 2002 that a sedentary lifestyle could very well be among the 10 leading causes of
death and disability in the world. 1 - Medications – drugs used to achieve glycemic control such as insulin, sulfonylureas or
thiazolidinedione therapy is generally accompanied by weight gain. Weight gain is also
common in with some atypical antipsychotic drugs (clozapine, olanzepine, risperidone and
quetiapine) are known to cause marked weight gain. Antidepressants such as amitriptyline,
mirtazapine and some serotonin reuptake inhibitors (SSRIs) also may promote appreciable
weight gain that cannot be explained solely by improvement in depressive symptoms. Mood
stabilizers such as lithium, valproic acid and carbamazepine as well as antiepileptic drugs
(AEDs) valproate, carbamazepine and gabapentin have all be found to promote weight gain.
Lamotrigine, however is an AED that is weight-neutral, while topiramate and zonisamide
may induce weight loss. 2 - Diseases such as hypothyroidism , insulin resistance, polycystic ovary syndrome , and
Cushing’s syndrome can lead to obesity. Many Polycystic Ovary Syndrome patients are
obese because they have the insulin resistance gene. This means that they are unable to
metabolize carbohydrates and sugar efficiently.
With a series of baseline tests, to uncover the cause of obesity, tried and tested methods for weight reduction can be implemented, which can make one drop at an average of 20 pounds every 40 days. Yes, it’s possible!
Reference:
- http://www.who.int/mediacentre/news/releases/release23/en/
- Ness-Abramof R , Apovian CM . Drug-induced weight gain. Drugs Today (Barc). 2005
Aug;41(8):547-55.
Vaginal Rejuvenation
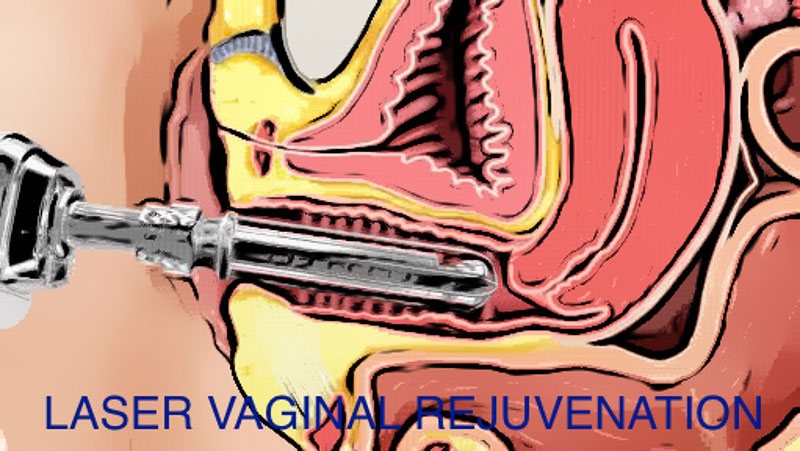
For women who undergo vaginal childbirth, as the baby passes the birth canal, the extreme stretch can cause the muscles, ligaments and fascia within the birth canal may weaken. This weakness may be exaggerated with subsequent vaginal births and especially with menopause, when collagen deposition drops with the drop in estrogen, leading to decreased vaginal muscle tone with age. As a result some women experience relaxed vaginal outlets, decreased sensation and sexual pleasure, vaginal flatus and a loose, gaping and embarrassingly unattractive vaginal opening. A host of changes of the vulva, vagina and lower urinary tract can occur leading to vaginal dryness, burning, irritation, lack of lubrication, painful intercourse, as well as urinary symptoms of urgency, painful urination and urinary tract infections. Vaginal discomfort and urinary incontinence can also occur in pre-menopause and after childbirth. Local estrogen treatment may be effective to treat vaginal changes. However, estrogen therapy is contraindicated for women with a personal history of breast cancer or estrogen-dependent tumors. For such women, as is for most women, laser vaginal rejuvenation can be an effective solution.
Fortunately, there are solutions to reconstruct the damaged muscles and tissues of the vaginal cavity to decrease the internal and external vaginal diameters. Laser vaginal rejuvenation restores the vagina and its supporting structures to a more attractive, “pre-pregnancy” state.
Laser vaginal rejuvenation is a breakthrough, minimally-invasive outpatient procedure that incorporates CO2 laser technology to effect optimal results to firm up your vagina, promote lubrication in cases of vaginal dryness and address involuntary passage of urine, called incontinence. The procedure involves inserting a fractional CO2 laser about four to six centimeters into the vagina. Upon firing, the laser beam penetrates about 0.5 millimeters into the vaginal wall, deep enough to reach the level of the skin where collagen is formed. This creates micro-lesions that are required for the production of new collagen which reorganizes and re-equilibrates the components of the vaginal mucosa and the urogenital structures, which enhances sexual pleasure, restores tone to tissue, and increases blood flow.
This, in turn, increases lubrication and strengthens the supporting ligaments surrounding the bladder and urethra to reduce the symptoms of stress urinary incontinence. It is a painless solution lasting only around 10 minutes. It is recommended that women undergo the vaginal laser procedure once a month for three months per year.
Hymenoplasty
It is possible to repair the hymen to a “virginal” state for those whose hymens were torn by any trauma like a straddle injury or activities such as ballet or horseback-riding or even sexual intercourse. It can be restored to a pre-sexual state. A woman can even unknowingly tear her vagina during the application or a tampon or during rigorous sports.
There may be cultural, ethnic or religious reasons to desire a hymenal repair. A history of sexual abuse may also warrant a hymenal repair as part of restoring a woman from her physical and psychological trauma.
Labiaplasty
Many women experience discomfort because of excessive flaps of the labia minora that protrude way beyond the labia majora. This results in great discomfort and sometimes, outright pain, when the flaps get caught in tight fitting clothes, or napkins when they have their menses or even during intercourse.
A reduction labiaplasty can address the problem and restore the confidence of the woman. Surprisingly, in my practice, majority of those seeking this remedy are virgins who are suffering from the discomfort or are embarrassed about the inequality of the left and right labia. Some women express they prefer their labia minora to be hidden by the labia majora.
Labial Tightening
Just like the face, the skin of the labia majora sags and wrinkles after repeated childbirth and especially after menopause, when the collagen deposition decreases with the drop in estrogen. This can affect a woman’s confidence during moments of intimacy. Laser treatments can actually effect a “facelift” to tighten the skin, adressing the layer where the skin and fat meet. As heat increases, the deep collagen in the skin begins to contract, causing a tightening effect with nearly no pain and no downtime for the patient. This results in a visibly improved appearance even after the first treatment.
Patient Testimony from Rocel Villegas

 “A BEAUTIFUL GOOD DOCTOR with A BEAUTIFUL HEART”
I am Rocel Villegas, 46 years old and a mother of 2. I have been a patient of Dra.Rebecca Singson since 1998 due to my polypses and I have known her as the best OB Gyne and beautiful doctor who is always very accomodating and treated all her patients with love and kindness.
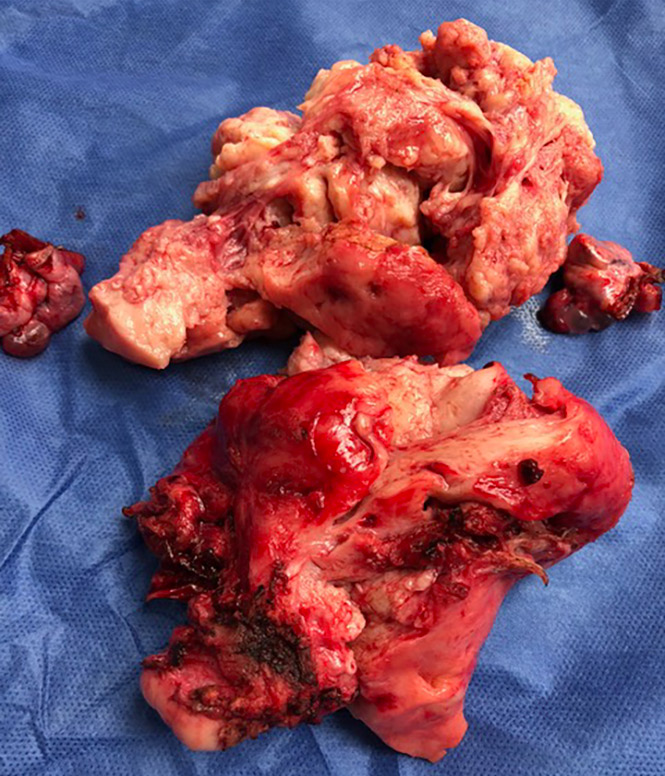
For the past few years, I had been gaining weight, experienced constant vomitting and abdominal cramps during menstruation. In December 2017, I was diagnosed with having multiple myomas, 2 of them measured 8.5 x 7.5 cm. I talked to Dra. Singson with all sincerity and honesty that our family is experiencing financial difficulty for four years now because my husband lost his business and my 11 year old daughter is under medication and counselling due to depression. Dra. Singson told me to find a way to pay for the hospital and other expenses for the surgery but not to worry about her professional fee. Her compassion and generosity touched my heart and brought tears to my eyes. On March 27, 2018, the surgical procedure I needed was successfully done by Dra. Singson at Saint Luke’s Global City and I am now on my way to full recovery. Dra. Rebecca Singson is Godsend and I am very fortunate to have her as my doctor. Our family will always be very grateful to her and will never forget her genuine kindness and generosity.
“A BEAUTIFUL GOOD DOCTOR with A BEAUTIFUL HEART”
I am Rocel Villegas, 46 years old and a mother of 2. I have been a patient of Dra.Rebecca Singson since 1998 due to my polypses and I have known her as the best OB Gyne and beautiful doctor who is always very accomodating and treated all her patients with love and kindness.
For the past few years, I had been gaining weight, experienced constant vomitting and abdominal cramps during menstruation. In December 2017, I was diagnosed with having multiple myomas, 2 of them measured 8.5 x 7.5 cm. I talked to Dra. Singson with all sincerity and honesty that our family is experiencing financial difficulty for four years now because my husband lost his business and my 11 year old daughter is under medication and counselling due to depression. Dra. Singson told me to find a way to pay for the hospital and other expenses for the surgery but not to worry about her professional fee. Her compassion and generosity touched my heart and brought tears to my eyes. On March 27, 2018, the surgical procedure I needed was successfully done by Dra. Singson at Saint Luke’s Global City and I am now on my way to full recovery. Dra. Rebecca Singson is Godsend and I am very fortunate to have her as my doctor. Our family will always be very grateful to her and will never forget her genuine kindness and generosity.