Management of Uterine Fibroids
For over 100 years, surgery was the mainstay of treatment of uterine fibroids or myomas. Up until recently, therapeutic management of symptomatic uterine fibroids was broadened to three pillars: surgery, pharmacotherapy, and interventional radiotherapy. Nearly 50% of hysterectomies done for benign reasons are due to myomas.1
American data reveals that myomas occur in about 60% of African-American women and in upto 40% or Caucasian women aged 35. The incidence goes even higher at age 50 to around >80% in Black women and 70% of Caucasian women. Sex hormones have been implicated to cause the growth of myomas.2 Thus, when a woman is approaching menopause which is at an average of 49 or 50 among Filipinos, the drop in hormones may actually save her from hysterectomy depending on the size of the myoma and severity of symptoms such as bleeding and pain.
SIGNS & SYMPTOMS. Signs and symptoms of myomas vary. More than size, location of the myomas in the uterus vary since very large myomas up to 16-20 cm located at the outermost layer of the uterus called the serosa, have gone unnoticed, especially if the patient is obese. Conversely, even a 1 cm. myoma can cause vaginal bleeding if located in the cavity of the uterus. The patient may also experience pelvic pain if the myoma causes pressure on the nearby organs. If a bulky tumor grows forward and applies pressure on the urinary bladder in front it, the patient may experience frequency of urination, or inability to completely empty the bladder or inability to sleep due to constant bathroom trips. If the bulky tumor presses on the back of the uterus, it can impinge on the intestines and cause constipation. Myomas may also cause infertility and frequent miscarriage. Myomas or polyps are found in nearly 30% of women with recurrent miscarriage. 3,4
DIAGNOSIS. The gold standard for diagnosis is still the transvaginal ultrasound for non-virgins, or transrectal ultrasound in virgins. However, if the uterus rises above the pelvis and may be too large for the vaginal probe to reach, a pelvic or transabdominal ultrasound is done. A Magnetic Resonance may also help visualize the position, relations to the adjacent structures and actual number.
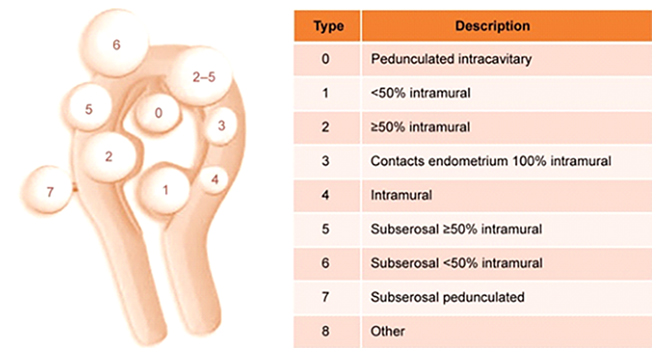
Figure 1
The International Federation of Gynecology and Obstetrics classification system for uterine fibroids.5
FIGO classification of uterine fibroids according to Munro et al. (2011). Fibroid types range from 0 to 8. 0 = Pedunculated, intracavitary; 1 = Submucosal, <50% intramural; 2 = Submucosal, ≥50% intramural; 3 = Contact with endometrium, 100% intramural; 4 = Intramural; 5 = Subserosal, ≥50% intramural; 6 = Subserosal, <50% intramural; 7 = Subserosal, pedunculated; 8 = Other (e.g. cervical, parasitic). Where two numbers are given (e.g. 2–5), the first number refers to the relationship with the endometrium, while the second number refers to the relationship with the serosa; e.g. 2–5 = Submucosal and subserosal, each with less than half the diameter in the endometrial and peritoneal cavities respectively. Fibroid classification cartoon republished with permission from Munro et al. (2011).
CONSERVATIVE MANAGEMENT
Patients who present with bleeding were usually offered tranexamic acid for 3-4 days monthly. It is a drug that promotes coagulation that has has been proven to reduce blood loss during menstruation. It is very well tolerated with few side effects.6,7.
The levonorgestrel intrauterine (LNG-IUS) has also been used successfully in bleeding patients with myomata.8 However, the presence of a submucous myoma distorting the uterine cavity may prevent a gynecologist from actually inserting the device properly.
Nonsteroidal anti-inflammatory drugs (NSAIDs) are also commonly used especially for patients with pain/and or bleeding in addition to hormones, either estroprogestins or progestogens, acting on the endometrium. Although it does not directly affect the fibroid to shrink it, it can reduce menstrual blood loss in women with mild and even severe bleeding. It is well tolerated as long as taken with food.9
Until 2012, GnRH agonists were the only therapeutic drugs approved for the treatment of myoma. The use of GnRH analogues reduces fibroid size and
bleeding symptoms. They act by reducing estrogen concentrations in the blood serum to postmenopausal levels. The side effects may be undesirable since it causes menopausal symptoms like hot flushes, skin and vaginal dryness, mood swings, loss of libido, vaginosis, depression, and bone loss. GnRH agonists are usually administered as subcutaneous injections for 3-4 months prior to surgery and they are not recommended for long-term use.
However, GNRH agonists have been losing ground because of their side effects such as hot flushes and bone loss plus the advent of a newer, cheaper drug that can be taken orally instead of subcutaneous injection. 10The new drug is ulipristal acetate (UPA), a selective progesterone receptor modulator oral drug, which can cause the bleeding from myomas to stop after 7 days of use and up to a 51% reduction in fibroid size after 13 weeks of use. By giving UPA tablets prior to Laparoscopic or Robotic surgery of large masses, shrinkage of the mass may actually happen with less possibility for anemia and blood transfusion since the bleeding pre-operatively may also be stopped. Another advantage of this drug versus the GNRH agonists is that the blood levels of estrogen remain higher similar to the estrogen levels women have in the first half of the menstrual cycle. This therefore means that there are less side effects such as thromboembolism and osteoporosis which are inherent to GNRH agonists. Side effects with multiple treatments of UPA are usually headaches, nasal congestion and sore throat, abdominal pain, and hot flushes. Because a greater number of
progesterone receptors have been found in myomata compared to normal uterine tissue another advantage of UPA is that it induces cell death or “apoptosis”. Therefore, unlike GNRH agonists, where the myoma shrinks only while you are taking the drug then grows back again to the previous size when you stop the drug, with UPA, the fibroid shrinkage persists for 6 mos. after stopping the drug. UPA, however, causes thickening of the lining of the uterus. Therefore, the drug is administered for three months then rest for two months before resuming the drug for three months again. 12
However, following recent reports of 4 women who developed severe liver failure while on UPA, 3 ending up with a liver transplant, on February 2018 the Medicines & Healthcare products Regulatory Agency (MHRA) advised of new temporary safety measures for ulipristal acetate13 following reports of serious liver injury in women using the medicine for uterine fibroid
The temporary safety measures are:
- Not to initiate new prescriptions for ulipristal acetate even for women who have already one or two courses of the drug previously.
- Monitor all women on the drug with liver function tests at least once a month. Stop UPA treatment in any woman who develops transaminase levels more than 2 times the upper limit of normal, closely monitor and refer for specialist hepatology evaluation as clinically indicated. Repeat liver function tests in all women 2 to 4 weeks after stopping treatment.
- For any recent or present users of UPA with signs or symptoms suggestive of liver injury (such as nausea, vomiting, malaise, right hypochondrial pain, anorexia, asthenia, jaundice), check transaminase levels immediately, If transaminase levels are more than 2 times the upper limit of normal, stop treatment, closely monitor and refer for specialist hepatology evaluation as clinically indicated.
- Advise women using UPA on the signs and symptoms of liver injury.
UTERINE ARTERY EMBOLIZATION
Uterine artery Embolization is a procedure where small polyvinyl particles are delivered via a catheter to the uterine arteries inserted on the femoral artery by an interventional radiologist. By doing so, the blood supply to the uterine fibroid is blocked, resulting in shrinkage, not disappearance of the myoma. It is indicated in women who have a major contraindication to undergo a surgery, or in women who strongly want to keep their uterus. Although it is considered a fertility-sparing procedure, it is, not recommended if a woman is desirous of pregnancy since she may actually drop her ovarian reserve.
Some of the side effects may include pain which results from occluding the blood supply, fever, infection and sepsis. The data also shows that if one resorts to a uterine fibroid embolization, the chances are higher that a patient may require a further surgery in two years which compared to women who underwent a straight myomectomy or an outright hysterectomy.14
HIGH INTENSITY FOCUSED ULTRASOUND (HIFU)
In contrast to Uterine Artery Embolization, HIFU uses no radiation. Rather, it acts much like the principle of using the magnifying glass to focus sunlight and burn paper. Focused ultrasound makes use of an acoustic lens to concentrate multiple intersecting beams of ultrasound on a target. Each single beam can pass through tissues with little effect but at the focal point where the beams all converge, the energy can generate heat high enough to ablate fibroids and cause cell death, blast ovarian cysts and even get rid of kidney stones.
On Oct. 2004, the US FDA first approved the HIFU for treatment of symptomatic uterine fibroids. The HIFU has been shown to be a safe and effective morality in achieving symptomatic relief while avoiding the risks inherent to surgery or other invasive therapies.15 Upto 16-20% will require additional intervention. 13
SURGICAL TREATMENT HYSTEROSCOPY
Hysteroscopic enucleation of a myoma is possible if the location of the myoma is inside the uterine cavity, the submucosal type. The patient usually comes to the clinic with complaints of bleeding. In fact, 59.8% of patients with myoma who present with vaginal bleeding actually have a submucosal myoma.16 It may also be discovered in patients who cannot get pregnant and in those with recurrent spontaneous abortion.17,18 Patients who have submucosal uterine fibroids may be attributed to dysregulated uterine contractility.19
In 20% of infertile patients, intrauterine fibroids or polyps are diagnosed.17,20 After hysteroscopic resection of intrauterine myoma, pregnancy rates
reach about 50%21 and bleeding symptoms are resolved in 70-99% of cases.18
As previously presented, we go back to the International Federation of Gynecology & Obstetrics classification of myoma:
• Type 0: the myoma is located completely in the uterine cavity or pedunculated.
• Type 1: <50% of the myoma is located in the myometrium.
• Type 2: >50% of the myoma is located in the myometrium.
It is important to determine this in order to predict the possibility for complete resection of the myoma prior to the procedure. According to European Society of Hysteroscopy (ESH) in 2012, this is the percentage success rate for complete resection of a myoma:
• Type 0: 96-97%
• Type 1: 86-90%
• Type 2: 61-83%
The success of surgery & rate of complete resection correlates inversely with the size of the myoma.22 An infertile patient diagnosed with a submucous myoma, even if it is less than 1.5 cm in size and without symptoms, must be subjected to a hysteroscopic myomectomy because the chances of success in pregnancy are high. In dealing with Type 2 myomas larger than 4 cm in size, a higher level surgeon expertise and experience is required since the complication rate increases with longer operating time. Since fluid is constantly infused into the uterine cavity in the course of the hysteroscopic myomectomy, there is a risk for fluid overload that can be fatal if unmonitored. 23





